While both experiments were essentially failures because there was contamination before I achieved an adaptation of yeast, there were some interesting results from each trial that may lead to some interesting supplementary experiments.
This experiment was interesting for 2 reasons:
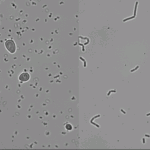
- The morphology of E. coli is different in different amounts of D2O. This applies to both colonies and individual cells.
- The growth of E. coli is almost completely uninhibited in the presence of 99.9% D2O.
While the links take you to pages that discuss yeast growth, it has been almost conclusively decided that there is bacterial contamination (most likely E. coli) and so the results shown above are not in fact yeast. A brief study must be conducted with my actual e. coli to compare that growth with the results from above. Although, the e. coli time trials of experiments past show exactly what the time trial linked above show: that for some reason E. coli really likes D2O.
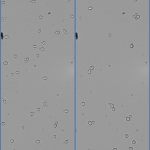
Also it was first noticed that yeast may not complete reproduction in 99% D2O, as there were a noticeable amount of “colonies”. These colonies are basically chains of buds that go unseparated. This analysis was repeated in…
While this experiment was relatively young, there was still a lot to be learned and a lot that can potentially be revealed:
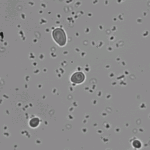
- Yeast morphology may change with increasing amounts of D2O.
The experiment only compared to yeast grown in 20% D2O vs 99% D2O, but the morphologies are clearly different. In 20% the cells were slightly elongated, while in 99% the cells are mostly round and pretty uniform in size.
Not only that but I also noticed that the bud chains I noticed in try 1 were more prevalent this time. The colonies had more time to develop (as the sample was 72h old) and grew into large clumps.