- I need a title for the paper. I’ve always called it Repeating Crumley, and maybe it makes sense to continue that trend, but is there a more fitting/descriptive name? Does it even matter?
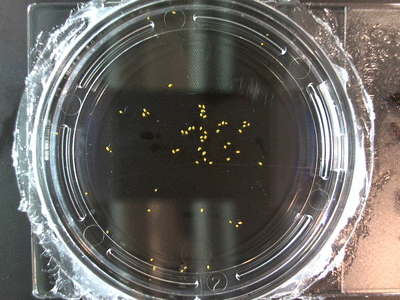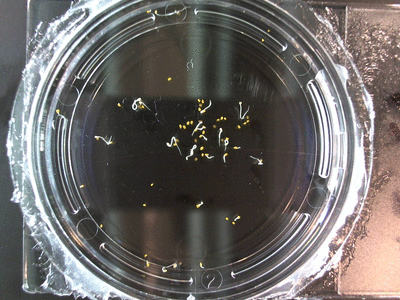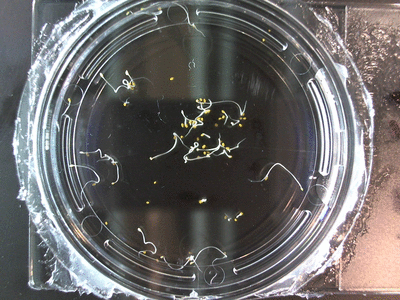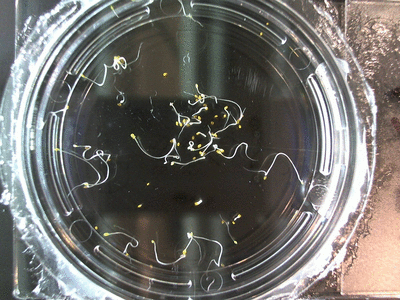
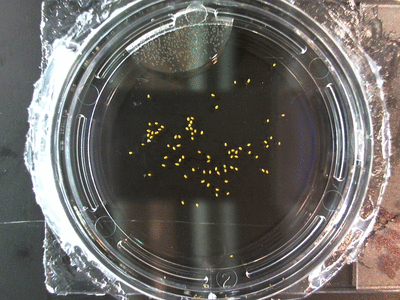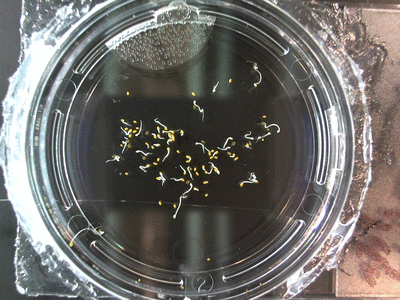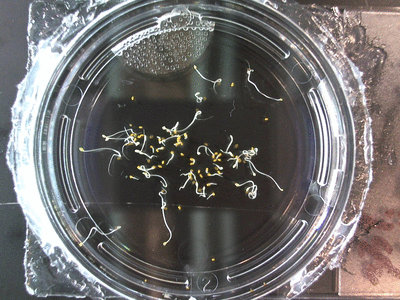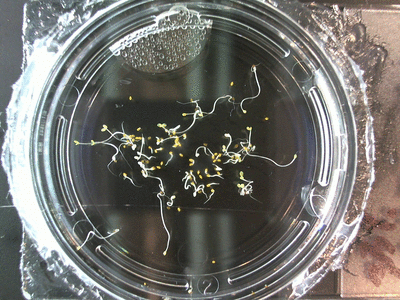
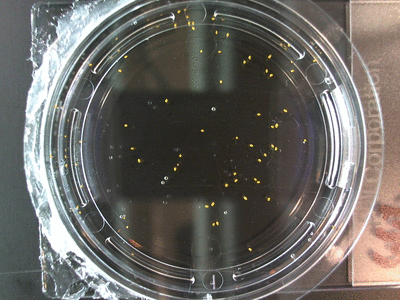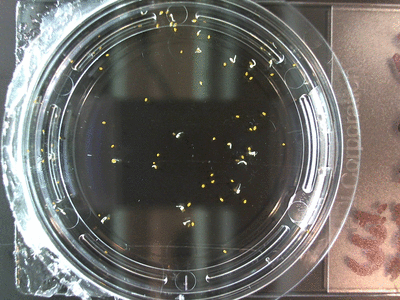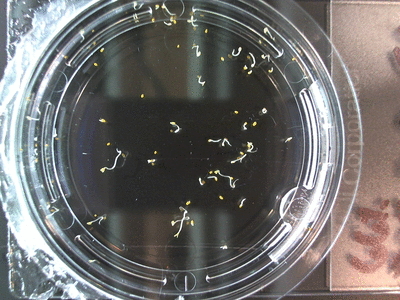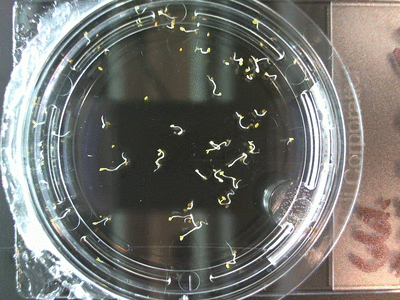
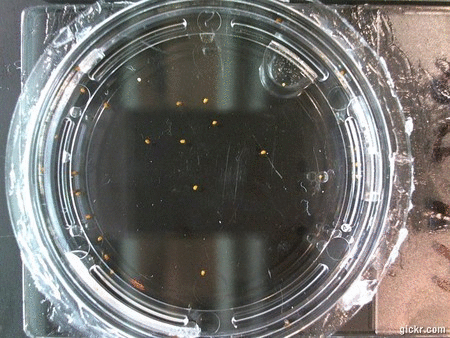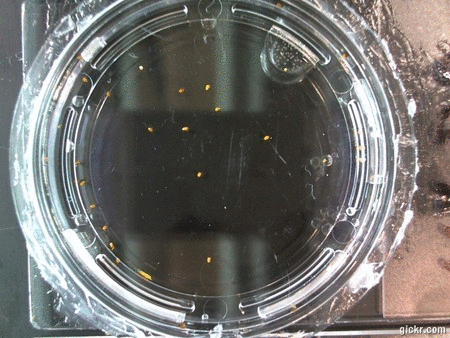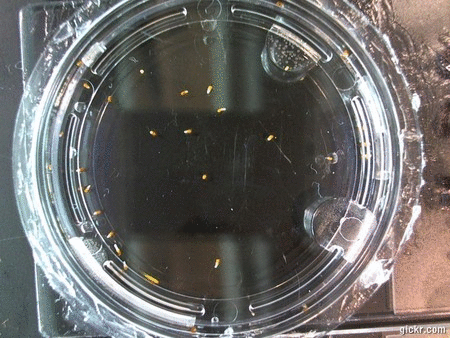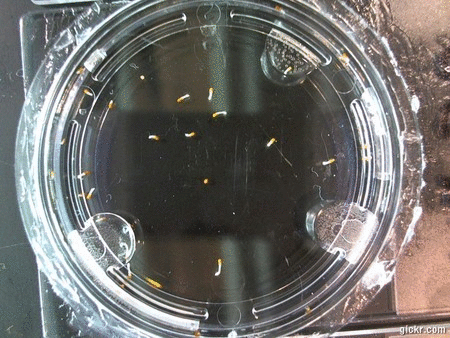
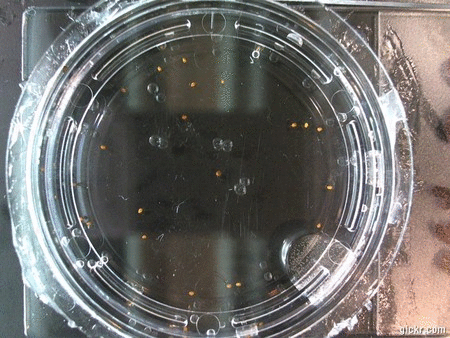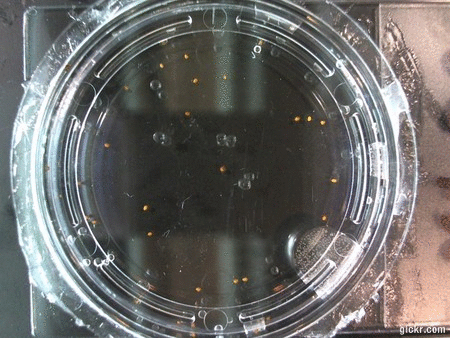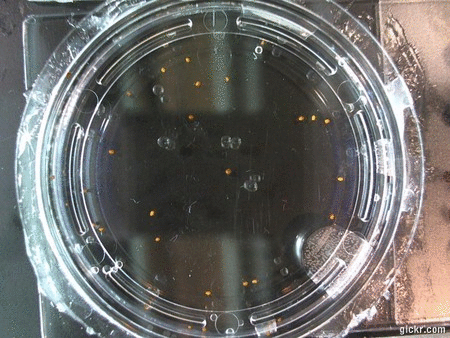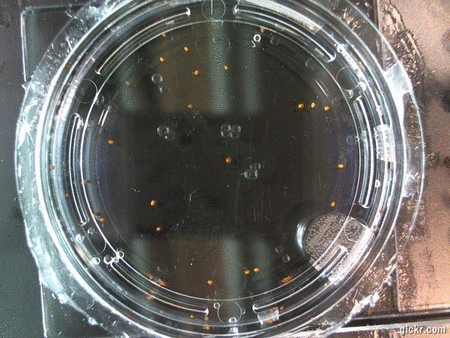
- I think it makes sense to create .gifs from all the plant germination images for each sample of each experiment.
- From RC1-4 I had slideshows, which allowed you to click through each sample at your own pace. Then after I had started making .gifs (especially since that was around the time of memes on the web).
- I still think it makes sense to have all the data as pictures as well. If they aren’t already there, I will upload all the images to figshare, and have a separate dataset as gifs.
- Should the gifs be stored via my notebook (and thus the Winnower), or figshare?
- Both?
- Since the Winnower can actively display the .gifs, this has my preference, but I’m not sure. Maybe both… just because.
- Making a citation list for every notebook entry may be tiring, but it must be done.
- I’ll have to go through my figshare profile to see what data is currently up there.
- I worry that I don’t remember some of the data analysis methods. I think the only one I have absolutely no recollection of is the root length vs time graph. I remember it happening but I don’t remember going from Point A to B. I think of this like getting in the car and driving to work. You remember getting in the car, but you have no recollection of the in-between time because you were lost in thought. This is what happens when your brain is in Dissertation/Defense mode.
- The primary focus on this paper is going to be about the replication of the Crumley experiment through my methods and the difference in our results. I will include some of the cooler data, but won’t be able to write a follow-up (yet) since there is insufficient data on some of the cooler experiments. But I can show preliminary stuff!
I think that’s all I got now. I’ll keep adding notes like this when I get more ideas, come across roadblocks, or something else.