DOI: 10.15200/winn.142722.25163 provided by The Winnower, a DIY scholarly publishing platform




DOI: 10.15200/winn.142722.25163 provided by The Winnower, a DIY scholarly publishing platform




DOI: 10.15200/winn.142801.10415 provided by The Winnower, a DIY scholarly publishing platform



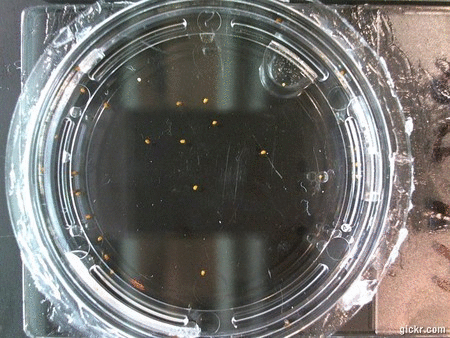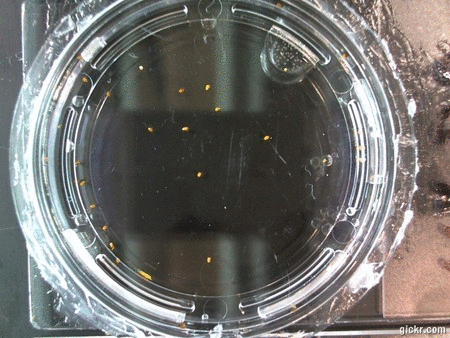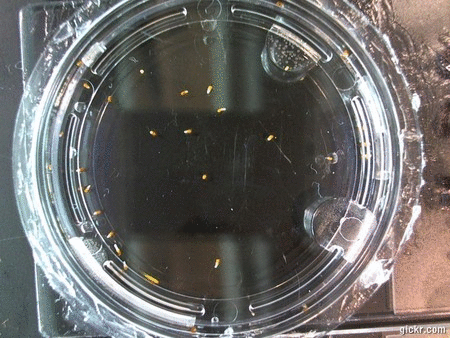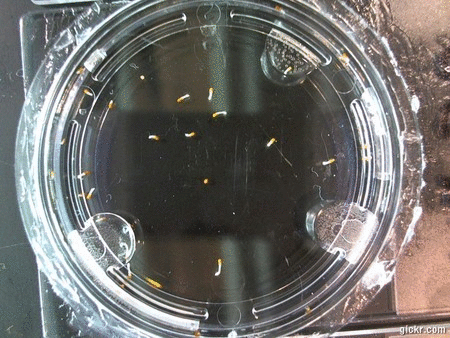
DOI: 10.15200/winn.142722.25159 provided by The Winnower, a DIY scholarly publishing platform
Just over a week away now…
Well there is my idea of how to present my dissertation. I’m not sure if/where I should put my discussion on open notebook science. Also there are a couple things that I could see going elsewhere. I could describe the yeast and e. coli stuff in parallel instead of one after another. Also the HD exchange stuff could easily go right after the yeast, e. coli, or even the tobacco seed stuff. What to do…
Otherwise I think the story is pretty compelling: history of D2O and the unanswered question by Lewis. Investigations into D2O effects and trying to understand low D2O concentration effects, effects on macromolecules, and the understanding of large volume/long-term HD exchange.
Any feedback you may have would be GREATLY appreciated. I’ll send you a figshare t-shirt, or if you are XL, I’ll send you a hoodie (but I only have one).
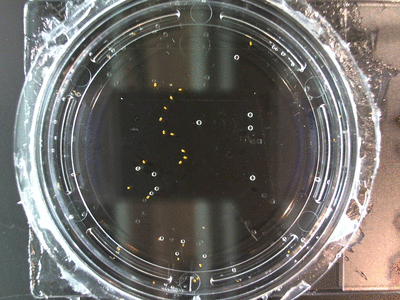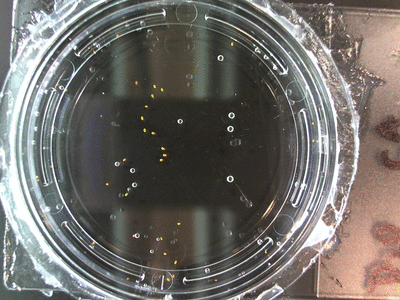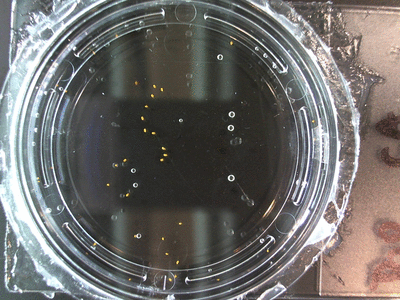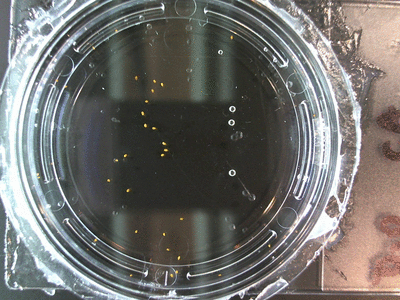
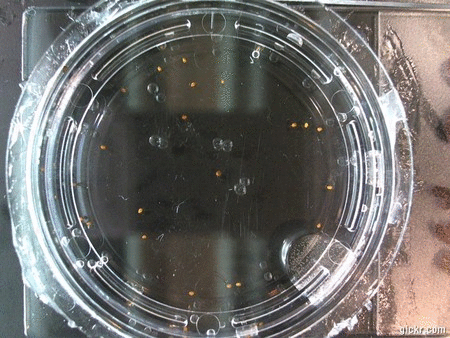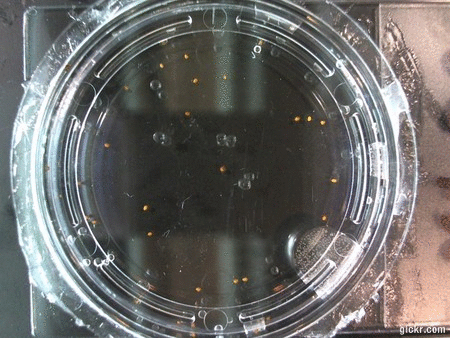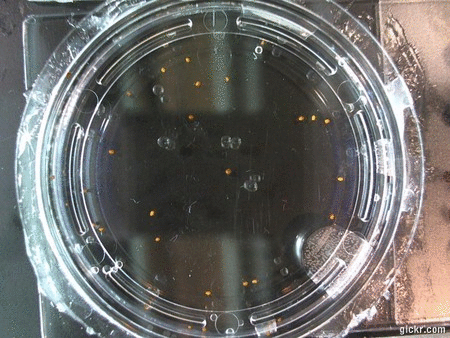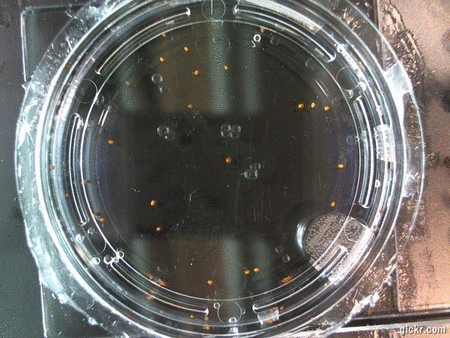
I got some awesome new data to show. The first is the compilation of all the Repeating Crumley experiments. And the second is some new data that I’ve been meaning to create and now have with the help of Koch.

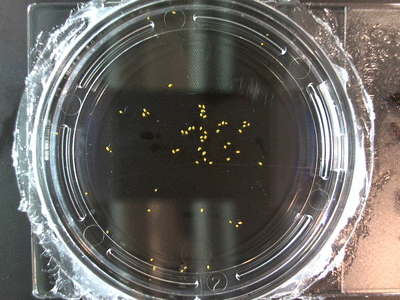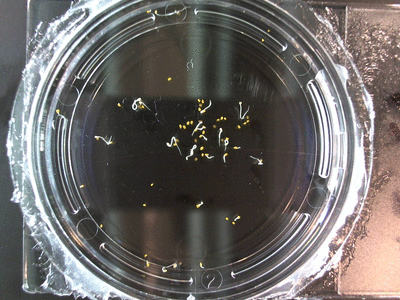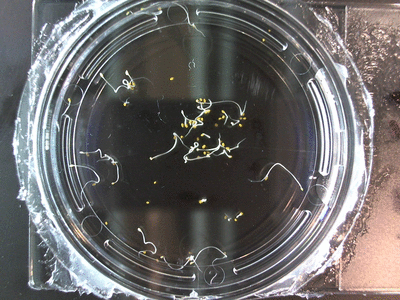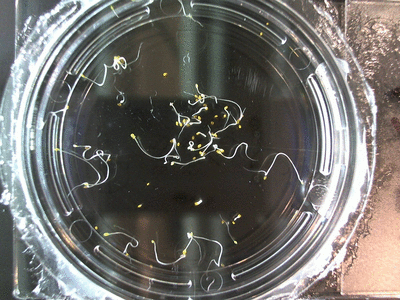
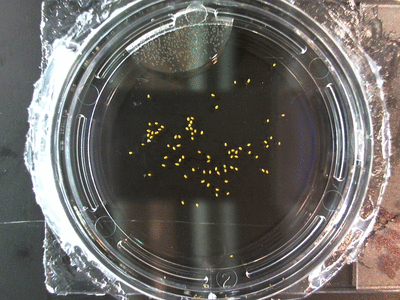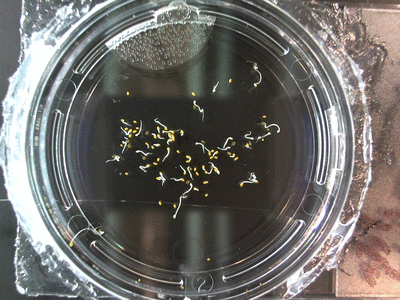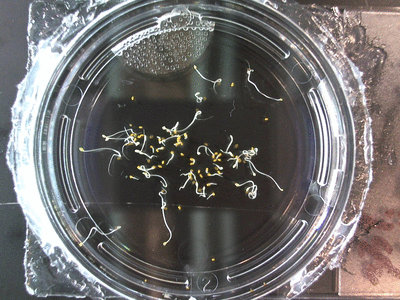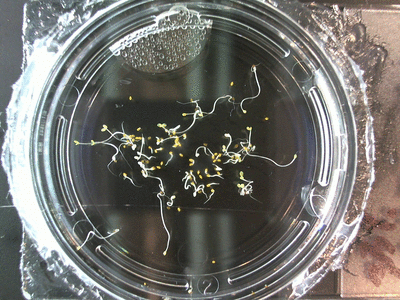
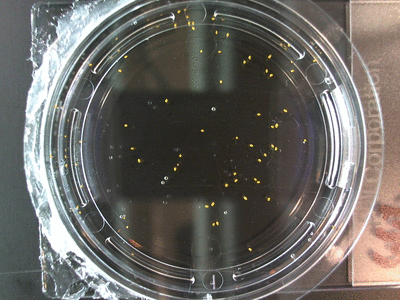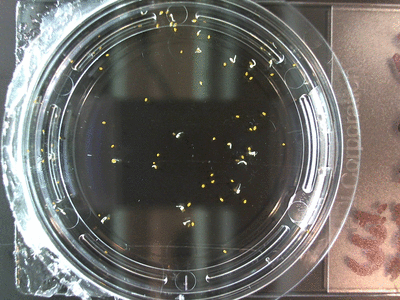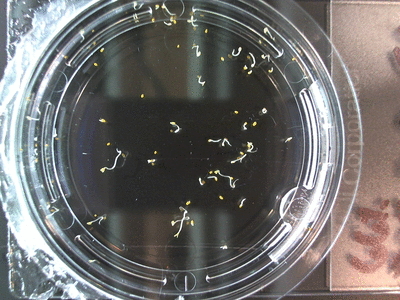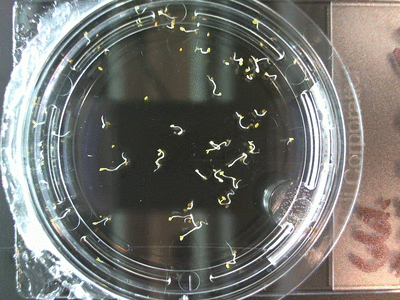
The data above is the compilation of all the RC data. Each trial had different water types, but I combined the samples that were the same in every set (DDW, DI water, 33% D2O, 66% D2O, and 99% D2O). Steve adapted his R-code that applies binomial confidence intervals to a data set and used it on this data. If that makes no sense, then just know that the dotted lines are the most probable range of germination rates. For instance, in 66% D2O there is a ~70% likelihood that seeds will germinate at a rate within the dotted yellow lines.
Now it’s time for some brand new data:

Here we went through the pictures from Trial 5 and compared the growth rates of the roots. We calculated the lengths of various seeds in each image and tracked the changes from image to image. We chose DI, DDW, and 1% D2O, because the D2O concentrations are relatively similar and because we wanted to test a hypothesis from a while ago. It’s interesting that the seeds in DDW and D2O grow at the same rate, while seeds in DI water grow at roughly half the speed.
H-D exchange (or D-exchange as I’ve sometimes referred to it) has been a problem I’ve dealt with in the lab for some time. It is essentially something that I need to minimize but can never actually stop. It is also a process that I know almost nothing about other than it happens, it occurs somewhat instantaneously, but may be mediated by evaporation rates (when dealing with DDW and 99% D2O). Now I’m perusing the internet looking for some information. Come take a walk with me:
Ok I’ve reached the limit of what I can do tonight without using the UNM network for access to papers. I’ll try to look for more information this weekend. The quest for a basic understanding of H-D exchange continues…
C. R. Bhatia and H. H. Smith, “Adaptation and Growth Response of Arabidopsis thaliana to Deuterium,” Planta, vol. 80, no. 2, pp. 176–184, 1968.
Looking for papers on yeast adaptation (of which I’ve found precisely one!) I came across this paper where they attempted to adapt arabidopsis to D2O. I was instantly intrigued and downloaded the paper for brain consumption. I will now take notes on the paper with the intent of setting up an experiment that can run with minimal effort on my part.
This paper must have been one of the last studies on organism development in D2O, especially because the papers that cite this paper are in unrelated fields and this is one of the most recent papers I’ve seen in this field. With that said, I’m a little skeptical of the effort to obtain D2O adapted arabidopsis.
I am impressed that they wanted to answer the question of whether H2O affects D2O adaptations in similar ways that D2O affects H2O adaptations. I don’t think their study was thorough enough and growing plants in 50% D2O still leaves the room for plants to get the H2O requirements. I think growth at levels above 70% would be key to obtaining true D2O adapted plants.
Their methods seem pretty simple and I could work on my own version of this experiment pretty easily and have already looked up product information regarding plant growth in the lab:
I’ll order those things tomorrow morning. And tomorrow I read a paper about D2O-Yeast adaptation!
I have no idea when this will get here, but I put in an order for some new S. cerevisiae. I think on Monday I’ll have Alex grow another starter culture and then on Tuesday, we’ll try and get better resolution under the microscope than we got last time.
Anyways, here is what I’ve ordered (from ATCC.org).
E. Coli Growth over 4 hours. Anthony Salvagno, Alexandria Haddad. Figshare.
Retrieved 20:17, April 20, 2012
hdl.handle.net/10779/b627469dabcd4034053cc53040d4dcbd
I went through the data that I posted on Tuesday and realized it was even less useful to people than I expected. I almost didn’t even know what I was looking at! Anyways, I did a couple of plots in excel with the data (which can be found on FigShare along with both the original data and the revised and cleaned data) and tried to extrapolate some other information. But first let’s discuss the experimental setup.
So on Monday, Alex created a starter culture from the E. coli we grew on plates last week. Then on Tuesday (I realize how not very real-time this post is for me, but the data came out in real-time which is also important) we made 3 dilutions of the starter culture to track the growth of the E. coli over time. We did:
Every hour we took an absorbance reading from the nanodrop and read the 600nm value (A600 according to the machine). We also reblanked every hour according to the instructions from the nanodrop. The initial readings were:
It is interesting to note (and I literally just noticed this), that the initial readings are almost exactly what the dilutions are, ie 0.097 is ~1/10 of 1.076. Good for us!
In the FigShare data, you will find the original data (which I linked to in my post on Tues, but in Google Docs instead of Excel) and a revised data set. The data is messy but the graphs are interesting.
Also I tried to link the 3 data sets together into one coherent graph, but the time series doesn’t seem to match up right, or maybe it does and I just think it doesn’t. The 1:5 dilution seems to provide a bridge between the data in the 1:10 dilution and the 1:2 dilution. After about 2 hours the 1:10 sample overlaps with the 1:5 and likewise the 1:5 begins to overlap with the 1:2. Also at hour 3, the 1:10 sample overlaps with the 1:2 sample.
Because of this I tried to graph the data as one continuous set. It seems Like it may be alright, but I feel that the in the 1:2 sample there isn’t much growth in the 4 hours, which is reflected in the 3 data set plot, but it doesn’t look like it peaks in the continuous graph. Hmmm… Anyways check out the FigShare data.
PS I’m including the two plots I’m referring to below.