[portfolio_slideshow]
Water Evaporation from Analyslide: DOW Corning Vacuum Grease Seal
[portfolio_slideshow]
[portfolio_slideshow]
I noticed an issue with the water evaporation rate from the sample chambers after the first trial. At first it wasn’t so bad, but got progressively worse. When the second trial came there was a heat wave in the lab (chiller went down for a week), and the evaporation rate increased. I discarded the results anyways because the heat affected the seed growth, but the evaporation rate would have ruined the experiment regardless.

Now I’m investigating the best way to seal the chamber while affecting the sample as little as possible. This is important because deuterium/hydrogen exchange could be a major source of experimental error and right now I have no real way of measuring this effect so it may be best to avoid it at all costs. I have purchased/selected sealants based on this. Also, based on the figure to the left, you can see it isn’t very easy to fit many things into that gap created by the top and bottom of the analyslide. The space there is 1/8″ so whatever I use has to either mold to the shape or be small. That is why I chose the following sealants:
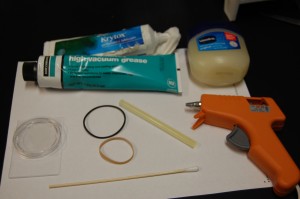
I filled 8 samples with exactly 6mL of DI water. I place the tops of the analyslides carefully on the slide portions and pushed them together slowly so as not to spill any water. I put pressure only on the walls and not the middle of the sample. I then put each sealant around the rim of the analyslide. For the vaseline, Krytox, and DOW Grease I used the giant q-tip seen in the picture to the left to spread the sealant around the area where the lid and the slide meet. For the o-ring and the rubber band I used a clean forceps to move the rubber over the same area. The glue gun glue filled the area between the lip on the top and the slide quite easily. For the parafilm, I cut a small strip (slightly larger than 1/8″ and stretched and wrapped it around the opening. I used to strips in hopes of ensuring a decent seal.
I chose these sealants for various reasons. I was looking for non-reactive “liquids” or suitable solids that could present the least amount of contact/transfer with water. Vaseline, Krytox, and DOW are all nonreactive (maybe not the Vaseline) and hydrophobic. Parafilm is waxy as well but is also a suitable solid. The rubber band and the o-ring were solid enough that I could place them wherever I wanted. The glue gun had the benefit of being a liquid at one point and a solid after cooling.
I was worried that the glue gun would heat the sample considerably but that wasn’t that big a deal. There was some sample heating but it wasn’t very much (although I didn’t measure the exact amount), and the glue cooled quickly.
My guess is the greases and the vaseline will work the best, but they are all the messiest. I don’t think the o-ring will work at all because it never seemed to make contact with the proper edges of the sample. But I have a feeling the rubber band may work well enough.
The experiment results can be seen “live” here.
So here I am at the midpoint of this experiment. Already the sample that was sealed with parafilm is completely evaporated. The sample with the o-ring is nearly empty. Meanwhile, the sample with no seal is doing really well. I’m guessing the parafilm somehow ruined the seal of the analyslide but in the case of the o-ring sample I’m thinking I didn’t seal that as well as I could have.
With that said, the rubber band is holding up particularly well. Obviously the greases (both vacuums and the vaseline) should seal well, and the glue gun as well. I will set up an experiment with a number of rubber band samples and see if they seal well over the next 8 days or so (once this experiment is complete). If they all do about the same then I will try to repeat the Crumley experiment with rubber bands as the seals. If not then I will just stick with vaseline or the DOW Corning vacuum grease.
Also it should be noted that I’m experiencing some issues with the slideshows I setup to track each sample change. On my home laptop (Macbook Pro, using Chrome) I don’t notice an issue, but here in the lab (Windows XP, using Firefox) not all the slideshows load completely (for me). And it is always the same slideshows that have the issue. If anyone else experiences this problem please let me know. Also of note, WordPress itself isn’t functioning properly so I’m guessing it is a Firefox issue and only on this computer.
And away we go with trial 3! I expect this version to go much better now that the lab temperature is regulated. Setup coming tomorrow when I have internet again.
DOI: 10.15200/winn.142722.20075 provided by The Winnower, a DIY scholarly publishing platform
It’s a new day and that means a new Crumley Trial. This is the 3rd try and hopefully one of the last trials (if everything goes smoothly). As always, I’m looking to repeat the results Crumley got back in 1960 growing tobacco seeds in D2O. Only I’m doing it better (no disrespect to Crumley, but there were some flaws in the original setup).
After getting the results of the water evaporation experiment I decided to go with the DOW Corning Vacuum Grease to seal the chambers. The product claims it has low reactivity with water and provides a great seal (according to my results). Since I want as little interaction with water as possible this seems like a viable option. With that said, let’s move on to the setup.
I prepared 8 samples for this trial using a combination of DI water, DDW, and D2O. I’m using Cuban Havana 2000 seeds from the Tobacco Seed Co (because the idea of growing Cuban cigar seeds in the lab sounds awesome!).
Sealing the chambers is quite a task that I have turned into an art. When I put the analyslide cover on I put it on loosely at first. Then I tilt it vertically so the air bubble moves toward the slight opening at the top. I begin to press around the edges with my thumbs until it is closed completely. Finally I spread vacuum grease around the rim (at first with the no cotton end of a long qtip, but then I used some plastic innoculating loops we had because it was thinner) for the final seal.
I will provide a post on the sealing process later today or tomorrow depending on who can show up to take pictures of me doing this.
DOI: 10.15200/winn.142722.20079 provided by The Winnower, a DIY scholarly publishing platform
For those counting along with me, the final counts won’t be out of 30. When I seal the chamber I end up crushing 1-3 seeds in the process and a couple more end up in the outer fringes where it is tough to discern sprouting or not. So I will count up the seeds in the interior circle (the fragmented one) and count the seeds that sprout from that total.
DOI: 10.15200/winn.142722.20083 provided by The Winnower, a DIY scholarly publishing platform
Here is the results after 24 hours. I haven’t looked in detail yet, but it’s interesting to note that there are 2 seeds in the 66% d2o in di water sample that appear to have sprouted already.
Also for those counting along with me, the final counts won’t be out of 30. When I seal the chamber I end up crushing 1-3 seeds in the process and a couple more end up in the outer fringes where it is tough to discern sprouting or not. So I will count up the seeds in the interior circle (the fragmented one) and count the seeds that sprout from that total.
DOI: 10.15200/winn.142722.20086 provided by The Winnower, a DIY scholarly publishing platform
So after the evaporation experiments from the past couple of weeks, I decided to go with the DOW vacuum grease as my sealant. Mostly because it is designed (supposedly) to be resistant to leaks and nonreactive with water, 2 major concerns regarding this project. I’m trusting the manufacturer and the performance of the product in the experiments on this one.

The analyslide has a lid and a base. The base has a circle on it with a slight gap (near the frosted part of the slide), which I’m pretty sure is what helps air escape (creating a decent vacuum) when putting the lid on the base. The top actually has two concentric circles. The inside one is perforated and aligns inside the circle on the base, while the larger circle on the top closes just around the outside of the circle on the base. Since there is no gasket or o-ring in the assembly, the seal isn’t ideal when dealing with water so further measures need to be taken.

After placing the lid on the base, I press the two together. I make sure to keep the analyslide verticle with the frosted side up (unlike what I’m demonstrating to the right). When there is water in the sample the air bubble that remains will align with the hole in the circle on the base of the slide. Doing this prevents water leakage when pressing the lid and base together. I ensure that I press firmly around the entire edge and evenly.
It is also in this step that one must be cautious. When there are seeds in the sample, they tend to float until water has soaked the seed coat. Because of surface interactions that are beyond my understanding at this time, the seeds also move around the surface and tend to migrate towards the edges. When placing the lid on, seeds may end up under the perforated circle or on the top of the base circle. This causes them to be crushed which could cause even more airflow into and out of the sample (because of the added thickness of the seeds).

Once the lid is placed on the base, you need to seal the chamber. Previously I used a q-tip because the handle was just the right thickness to fit between the lip on the lid and the base. I eventually switched to a plastic gamma sterilized innoculating loop given to me by a neighboring lab (thanks Nathan!). The loop is a little thinner than the q-tip and I feel allows me better control over sealant application. When sealing, I actually don’t use the loop part at all, and rather use the other end (smearer?) but I’ll still refer to it as the loop for this explanation.

I get a relatively large (but thin) amount on the end of the loop and spread it around the lid-base interface. I make sure to get the majority of the sealant near the gap between the two to ensure a proper seal. Pay extra attention when applying near the sides of the base, because here the lid protrudes a bit and so the sealant must actually be placed near the underside of the slide.
For your viewing pleasure, I am including all the pictures I took (that aren’t too blurry) for this demonstration. I’d like to thank Nadia Fernandez Oropeza for help in taking the images you see here.
I forgot all about taking pictures for Day 2. So I took them this morning and we should really call them Day 2.5. I’ll post Day 3 later today to keep us on track.
DOI: 10.15200/winn.142722.20091 provided by The Winnower, a DIY scholarly publishing platform
DOI: 10.15200/winn.142722.20096 provided by The Winnower, a DIY scholarly publishing platform