There are 5 seeds in each sample, but some seeds may still be floating. Hopefully the seeds will have settled by tomorrow so I can take better pictures.
Tag Archives: ddw effects on life
How Much D2O is in Natural Water? UPDATED
Update: I realized that I was off by a factor of 10 in my calculation for the amount of D2O to add to DDW. It turns out that the amount you need to add is 0.14ul of D2O per 1mL of water, which is vastly different from 1.4ul.
I was hoping to start the third attempt at the “Effects of DDW on Tobacco Seeds” experiment but got caught up doing some “trivial” calculations. It’s hard to do trivial things when you haven’t done math in a while. Why was I trying to do these calculations?
Well it occurred to me that perhaps the root hairs we saw weren’t a product of there being no D2O in water, it could have been as simple as thinking there was nothing at all in the DDW besides H2O. Perhaps this water is so pure that the plants are looking for something in the water and the hairs are a byproduct of that.
So I thought, what if I added enough D2O to the DDW so that it has the same amount of D2O as naturally occurring water. This way the only real difference between the ddw sample and the di water sample is how the waters were purified. Maybe the Thermo purifier water we have actually has trace amounts of something in it and this is why the hairs aren’t as prominent.
In order to set that up, I needed to know how much D2O is in natural water. The short answer turned out to be 156ppm (parts per million) D2O in H2O according to some standard from ocean water. And according to my calculations results in a concentration of 7.8mM, which is quite a staggeringly large number coming from a molecular biology background. Steve put it best in this blog post:
0.03% does sound trivial. But the way I look at it, biology has somehow evolved to make use of different divalent cations in much lower concentration, such as magnesium, zinc, calcium, etc. And it can distinguish between potassium (K+) and sodium (Na+). How much more different are K+ and Na+ from each other, compared to the difference between D and H?
So 7.8mM would translate to about .14ul of “pure” D2O in 1mL of “pure” deuterium depleted water. Of course we don’t have pure of either of those. We have 99.9% pure D2O (99.9 atom % D according to the bottle) and < 1ppm D2O in deuterium depleted water.
Now I became stressed! Can I assume that those amounts are so trivial in 1.4ul of D2O that I don’t need to worry about adjusting the calculations? Well I ran some other numbers to make sure.
99.9% pure D2O is roughly 1000ppm H2O (assuming H2O is the contaminate in the bottle), roughly 10x more concentrated than the amount of D2O in natural water. It turned out the concentration of H2O in my D2O was around 55mM. So in 0.2ul of D2O there would be ~2nl of H2O. An extra 2nl of H2O in 1.999ml of D2O is not much change at all.
What about the amount of D2O in the DDW? Well 1ppm turns out to be about 10nM which equates to about 0.9nl in 1mL of DDW. Again less than 1nl won’t affect (too much) the 0.2ul that I’m already adding. Instead of there being 2ul it will be 2.001ul. I think I can live with that!
Unless I come to the realization that all these numbers are important. If that’s the case then I’ll go back through and figure out exact amounts to add to offset the potential impurities in both solutions. Yay for me!
CHTM Cooling System Down
Since this past Friday (Sept 23, 2011) the cooling system at CHTM has been down. I’m not sure if it is turned off for the impending winter or temporarily broken. This means that right now the lab is a toasty 92F and has been that way all weekend long. Unfortunately this can potentially mean a lot with regards to my seed growth experiments. Now I’m not sure if this is actually the case, but I think with regards to the experiments it may be in the best interest to scrap Try 2 for both the Repeating Crumley and the DDW Effects on Seed Growth until the temperature becomes a bit more stabilized. Now I will still record data and keep track of the experiments, but I will not include these results in any final markups. But that doesn’t mean I can’t learn something from these experiment!
DOI: 10.15200/winn.142721.17155 provided by The Winnower, a DIY scholarly publishing platform
DDW Effects on Life Trial 2: Setup
This is the setup for the second trial of deuterium depleted water effects on plant growth. For the most part the experimental setup is identical to the first trial which you can find here. There are a few notable differences though so let’s run through the prep:
- 5 seeds of two differing varieties from The Tobacco Seed Company are used in each sample (trial 1 had 3 seeds per sample). I am using Dark Virginia and Virginia Gold #1 variety seeds.
- there are 5 different water types: 99.9% deuterium depleted water (ddw), deionized water (di water), tap water, 33% deuterium oxide (d2o) mixed with di water, and 33% d2o with ddw. The d2o mixtures are new additions to this experiment.
- The samples are stored in semi-micro cuvettes and sealed with a silicon top (maybe it’s rubber).
- The seeds are added to a cuvette and then 2.5ml of a water is added to the sample. This volume minimizes the air bubble in the sample to minimize hydrogen/deuterium exchange with the water.
- There are 10 samples of seeds and 10 more samples of seeds that are presoaked in their respective water buffers.
- Seed growth is tracked using a Nikon D40 DSLR with a 10x magnifying lens. The samples are arranged on some Thor Labs optomechanics to make image taking simpler.
DDW Effects on Life Try 2: Day 3
I’m going to shorten this title to DDW Effects 2: Day X and it should be noted that I won’t post every day. Just as things happen. It’s not critical for this experiment that we track growth or time. I’m just noting phenotypical differences in the development, like the hair. I’ll be posting a setup page soon and that will contain all the information pertaining to this project.
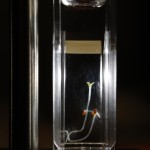
This first post is a picture of the seeds and some have already begun to sprout. I didn’t have the abilities to take Day 0 pictures because my station was torn to shreds (I was redesigning a way to easily take pictures of every sample for this and the RC experiment). Now everything is up and running and should proceed smoothly. Here are the pictures:
Some shorthand explanation: the caption format is “seed subspecies, water type”
- vg – virginia gold; dv – dark virginia
- ddw – deuterium depleted water; di – deionized water; tap – tap water; d2o – deuterium oxide
- “33% d2o in xx” means I mixed 33% d2o with xx water (either ddw or di water) by volume. For instance in 3mL of sample, 1mL is d2o and the other 2mL are the water type specified.
Videos of the Root Hairs
Video 1 – (Download by right clicking and selecting save as ~40MB) This is the dark virginia seeds in ddw as viewed from the front of the cuvette.
Video 2 – (Download by right clicking and selecting save as ~18MB) This is the dark virginia seeds in ddw as viewed from the side of the cuvette.
Video 3 – (Download by right clicking and selecting save as ~25MB) This is the dark virginia seeds in tap water as viewed from the front of the cuvette.
Video 4 – (Download by right clicking and selecting save as ~34MB) This is the dark virginia seeds in ddw as viewed from the front of the cuvette, but I’m wiggling the cuvette holder so you can see the motion of the hairs and differentiate them from scratches on the glass.
Follow Up on Yesterday’s Final Results of Tobacco Seeds in Water
Here are all the pictures as promised from yesterdays presults (like that one?). Compare the first picture in the gallery to this one from the last update 15 days ago. Notice the amount of growth in the past 15 days.
While the seeds in ddw have kept growing, it appears the seeds in all other samples have either stopped growing, are growing slower, or are dying. This time most roots did not have hairs or had very little hairs, which is different from last time where the hairs were more obvious. I don’t know what to make of this for now.
I’m also not going to make any conclusions or assumptions in this post or any other observations for that matter. I’ll leave it up to you to think about what’s different and post observations in the comments. The one thing I will say though is that this study is fascinating!
I took some videos of the hairs which I will post in the next post.
Final Update on Tobacco Seeds in DDW
Well oh well. Look at what we have here. I’m so excited I can hardly contain myself…
…ahem.
So it’s been a while since I updated and that’s because I’ve pretty much left the seeds to their own accord. I didn’t want to end the experiment officially because I wanted to see if there would be any other startling results, but it was pretty much over. Now I think I got some startling results. Let’s talk about this:
- I took pictures of 3 different samples, one dark virginia in ddw, one dark virginia in tap water, and presoaked dark virginia in ddw. The pictures are lit (to see the results) using my new Droid Bionic camera led (for flash) as the lighting source.
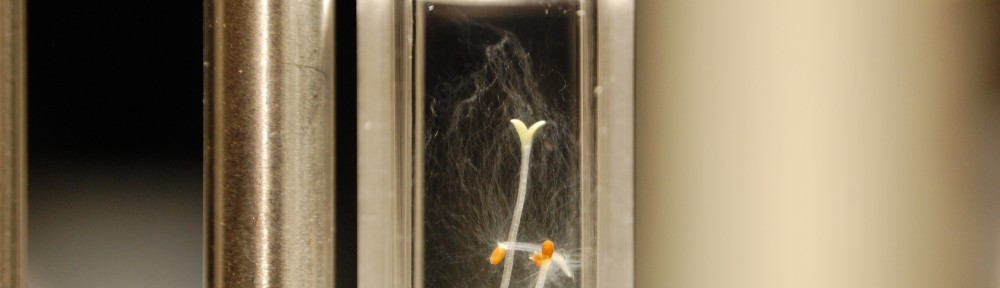
- Two pictures are of the same sample. One is very well lit and the other is dark, but with some side light (I liked the way it looked artistically so I included it and made it the feature image). In these two images I wanted to illustrate that the seedlings were fully germinated (and probably at max growth submerged in water), but that the root hairs I adoringly refer to as root fro almost have completely filled the solution! What the hell could this mean? I no longer think it is a lack of nutrients. Why? Because this effect is only seen in the samples of ddw. The DI water samples which never had quite as much root fro (but quite a bit) looks to have actually gone into remission, meaning the root fro seems to be getting less.
- To contrast that I showed what it is like in a typical sample of tap water. Note that there is root fro in there (the plant on the bottom has its hairs visible) but they are nowhere near filling the sample. Crazy!
- To make matters even more crazy, the presoaked seeds in ddw haven’t even germinated properly and there are (is?) root fro everywhere! Both presoaked samples are like this. On top of that it looks like presoaking may have hindered or impaired the germination process because every sample’s growth is stunted. The root fro amounts per sample is typical though (meaning ddw has the most, di water next most, and tap water the least amount of fro).
Holy moley! I don’t even know what to make of this. I’m going to re-setup this experiment in a few days but include D2O (like 33% and 66% amounts) to see if the root hairs are a similar phenomenon and to what extent.
All I can say is someone get me a molecular comb!
No Tardigrades in the Lightning Field
No tardigrades at the lightning field…
Well I’m not sure if that is true, but I couldn’t find any tardigrade habitats. The landscape of the Lightning Field was mostly plain (as in prairie, not ordinary). There was almost no tree or rock where I’m used to seen moss and lichen grow. It had been raining frequently so it was muddy, but I’m not in the business of bringing back dirt (and I don’t think my girlfriend would appreciate that too much either). Oh well for that…
Tardigrades in Quemado
After deciding to get some lichen and moss samples for tardigrade acquisition here in ABQ (at the Rio Grande) I decided to continue my efforts near The Lightning Field near Quemado, NM which I will be visiting for the next 24 hours. Now I have no idea what the terrain and conditions will be, but I feel like there is some chance I can find what I need.
In any case I’m going prepared. I’ve brought a few petri dishes, a razor blade (for removing samples from the wild), some latex gloves (for sterility and protection), and rubber bands (to keep the petri dishes closed). Hopefully I come back with something.
Map of Quemado, NM (You may need to zoom out)
Featured image courtesy of Wikipedia.