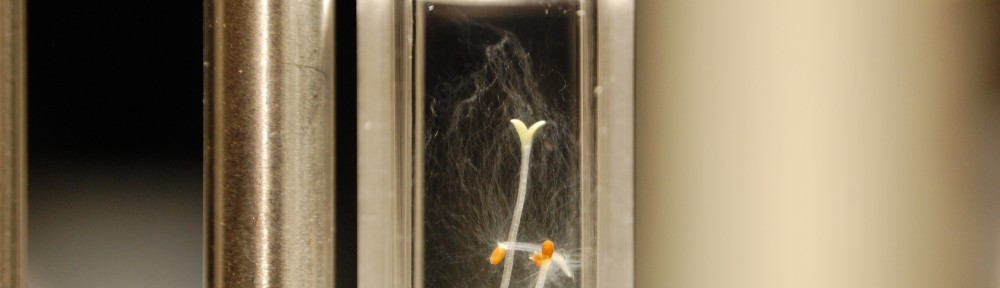
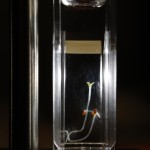
Since this past Friday (Sept 23, 2011) the cooling system at CHTM has been down. I’m not sure if it is turned off for the impending winter or temporarily broken. This means that right now the lab is a toasty 92F and has been that way all weekend long. Unfortunately this can potentially mean a lot with regards to my seed growth experiments. Now I’m not sure if this is actually the case, but I think with regards to the experiments it may be in the best interest to scrap Try 2 for both the Repeating Crumley and the DDW Effects on Seed Growth until the temperature becomes a bit more stabilized. Now I will still record data and keep track of the experiments, but I will not include these results in any final markups. But that doesn’t mean I can’t learn something from these experiment!
DOI: 10.15200/winn.142721.17155 provided by The Winnower, a DIY scholarly publishing platform