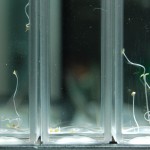
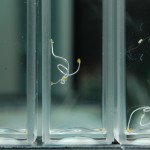
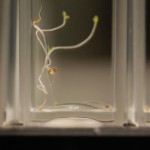
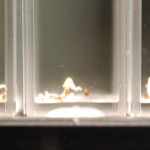
After 4 trials and talking with Steve, there is no way to ensure that the results we are getting have anything to do with deuterium content. We can’t tell at all that our DI water is just not the same kind of “pure” that the DDW is. So I bought deionized water from Sigma (same people who make our DDW and D2O) to compare with our water and to compare to the DDW results to determine if the root hairs grow based on water purity or if it has something to do with deuterium content.
Here is the setup of the experiment:
- I am using 4 different “types” of deionized water. We have a Easypure RoDI from Thermo (see Experiment Product Page at top) that I get DI water from. CHTM also has their own deionized water filtration system and I’m using that water in this experiment as well. I also purchased two different kinds of pure water from Sigma, one is molecular biology grade water and the other is double purified water for tissue cell culture. I honestly don’t understand the differences, but this chart says there are some.
- I also used pure DDW for one sample and a 1% D2O mixture with DDW for another (because why should I exclude the DDW results from this study?)
- I am keeping this experiment to just the tobacco seeds since the arabidopsis results are perplexing and not as obvious. So I am using both kinds of tobacco seeds (Havana and Virginia Gold #1, see Experiment Product Page).
- I chose to do four samples per water type per seed type. With 5-7 seeds per sample. That means for each water type there are 8 samples, 4 for each type of seed.
Today’s protocol was really easy. I used my macro cuvettes (see Experiment Product Page) and set up 4 cuvettes for each water type giving me a total of 24 cuvettes. I did this so I could set up samples for one seed type at a time, and because I only had enough racks for this many cuvettes. I also prepared the D2O/DDW mixture (29.7mL of DDW, 0.3mL of D2O).
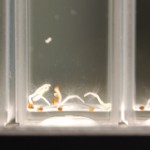
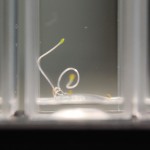
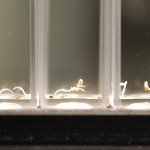
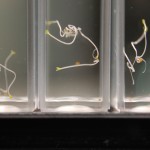
I poured 5-7 seeds in each macro cuvette until all the setup cuvettes had seeds in them. Then I added 3mL of water to the samples (again making 4 samples per water type). Then I sealed the cuvettes with PE caps.
I repeated this setup for the next seed type (24 cuvettes, 5-7 seeds per sample, 4 samples per water type, with 3mL of water per sample) and sealed and labeled all cuvettes.
I placed the cuvettes in the 4C fridge to synchronize the growth among all the samples. Tomorrow I’ll remove the seeds and take Day 1 pictures.