Hydrogen has several isotopes and one of them, deuterium, exists quite naturally in water to form 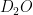 . In previous experiments and several papers by Gilbert Lewis, it has been found that life is hindered in the presence of
. In previous experiments and several papers by Gilbert Lewis, it has been found that life is hindered in the presence of 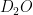 . While this may be true, my PI Steve Koch wondered if life had found a use for it because naturally occurring water has about a 17mM (millimolar) concentration of deuterium.
. While this may be true, my PI Steve Koch wondered if life had found a use for it because naturally occurring water has about a 17mM (millimolar) concentration of deuterium.
To put that number into perspective, when I do a typical polymerase chain reaction of DNA I add 10mM of each base of DNA (which is less than the amount of naturally occurring deuterium) to create millions of copies of a DNA template from an amount that is 1000x less then what the reaction yields. In fact most chemicals in most of my buffers on the order of the amount of naturally occurring deuterium.
So you can see it isn’t a stretch to think that nature has found a use for 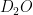 since it is quite abundant and life has been constantly evolving for billions of years. I want to test this hypothesis in a variety of different organisms:
since it is quite abundant and life has been constantly evolving for billions of years. I want to test this hypothesis in a variety of different organisms:
- Tobacco Seeds – to act as a foil to Lewis’ experiments in which he grew tobacco seeds in pure
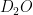 .
.
- Mustard Seeds – from what I’m told mustard seeds are the powerhouse of the botanical genetics world much like Drosophila and S. cerevisiae are in their respective genetic fields.
- Escherichia coli – another molecular biological powerhouse that is very easy to grow and may be easy to see results with. We just got the facilities to be able to grow E. coli and damn it I want to use them!
- Saccharomyces cerevisiae (Yeast) – I know a guy who grows yeast for his experiments and I’m sure it wouldn’t be a stretch to get him to do so in deuterium depeleted water.
So the idea would be to try to grow these in regular water and in deuterium depleted water (no 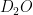 ), and in the case of E. coli and yeast, perhaps in pure
), and in the case of E. coli and yeast, perhaps in pure 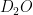 because I don’t think those experiments have been carried out yet. Hopefully I will be able to conclusively state whether or not life has developed a need/use for
because I don’t think those experiments have been carried out yet. Hopefully I will be able to conclusively state whether or not life has developed a need/use for 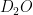 which would be a very interesting discovery indeed!
which would be a very interesting discovery indeed!
Like this:
Like Loading...
