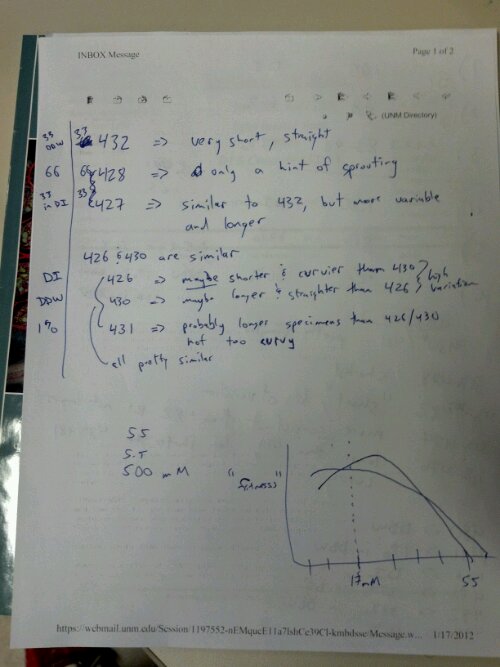
Steve and I are trying to come up with ways to quantitatively measure differences between seeds grown in ddw vs di water. Via the slideshows it is obvious that length is one such observable. Is mass another?
Perhaps, but it’s not easy to extract a number from such small samples. I gave it a try nonetheless. 2 days ago I opened all my sample chambers and allowed the water from each sample to evaporate and the plants to dry out. I used seeds from both the Repeating Crumley experiments and the DDW Effects experiments.
I realized quickly that I can’t weigh the seeds in the analyslides because the vacuum grease that I use to seal the chambers isn’t uniform on any of the dishes. That means that I can’t take a baseline weight of one slide and use that as a zero for all the dishes. I then threw out the seeds without realizing I could try and scoop out the plants and weight them on weigh paper or something. Next time.
The seeds in the DDW Experiments are contained in macro cuvettes. I took four empty (and new) cuvettes and weighed one and zeroed the scale. Then I weighed each of the others and found that two others were identical in mass and the fourth cuvette had a mass that was 0.0004g less than the original cuvette. Not bad.
I then began to weigh each of the cuvettes that contained dried plants.
BAD IDEA!
It seems the tobacco seedlings are so slight that every sample I measured was around 0.0003g lighter than the blanked cuvetter! There are on average 5 seedlings in each sample. So that means that 5 seedlings have less mass combined then the error associated with inequalities in the mass of different cuvettes. Interesting but sucky.
It is pretty hard to remove the seedlings from the cuvettes without destroying them so I’ll have to return to the drawing board. I think I will setup another RC experiment and scrape the seedlings out of there (weigh the seeds before adding them to the batch) at the conclusion of the data acquisition and weigh them via some other means (again probably on weigh paper).
Ideas?
Note: I tried to do this with the arabidopsis but realized that those seeds are definitely too light to be noticed by our supposedly super sensitive balance.
Like this:
Like Loading...