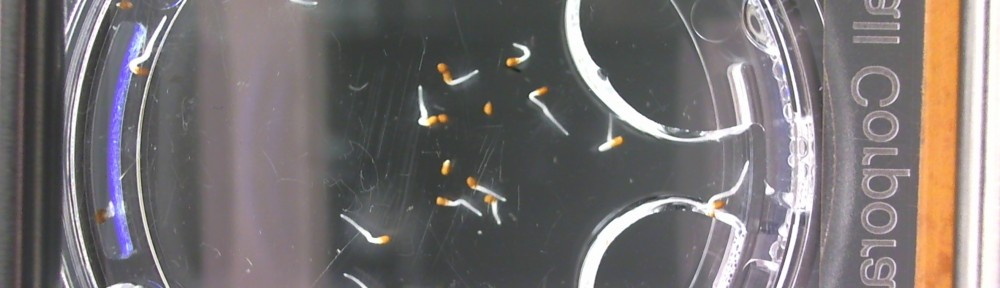
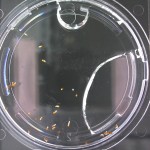
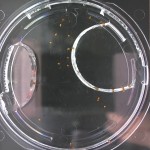
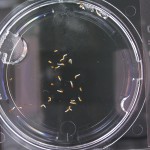
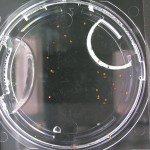
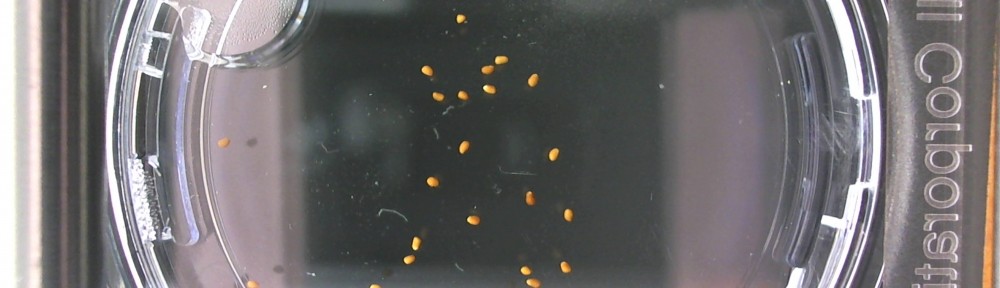
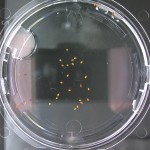
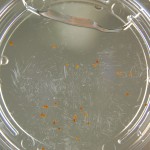
Here are the Day 7 results. Looks good so far. There are some troubles though. It looks like I have extensive leakage in the 66% D2O and 99.9% D2O samples, because each day the air bubbles get bigger. How can I fix this without restarting the experiment?
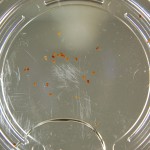
Also in the 33% D2O sample there is that white thing near the center and two unsprouted seeds. I have a feeling the white thing may either be from one of those two seeds. It could also be from a broken seed from the pack that was stuck to the tweezers and which I didn’t notice got added to the lot when I poured the seeds into here. What to do? I won’t know for sure until like Day 15 or so when most of the seeds in this sample would have sprouted. Ideas?
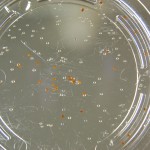
Finally you can definitely see the root hairs in the DDW sample and the DI water samples this time. Not so much in the 33% sample. In all honestly I can’t even tell by the naked eye. Hopefully this will be obvious by day 10. And as usual, nothing to report in the no seed sample.