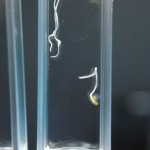
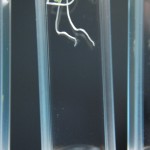
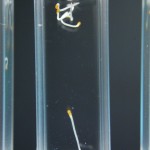
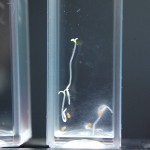
Didn’t expect that one did you? The purpose of this experiment is to study a much more narrow range of D2O mixtures based on the RC experiments. We want to determine if there is some amount of D2O that maximizes growth in tobacco seeds. If there were it would be somewhere between none and 5% in my opinion, and could quite possibly be right at the natural water amount of D2O (15mM).
So this experiment will be setup exactly like the RC experiments but we’ll be tracking length with some software after the images are acquired. Here is how I setup the experiment:
- There are 8 samples of tobacco seeds and each sample contains 35-42 seeds.
- The 8 amounts of D2O are:
- pure DDW (no D2O)
- standard mean ocean water (15mM D2O)
- 1% D2O in DDW
- 5% D2O in DDW
- 10% D2O in DDW
- 20% D2O in DDW
- 25% D2O in DDW
- 33% D2O in DDW
- I know the amounts are strange but I figured in following experiments I will do different amounts for a more robust data set.
Below is a table of the amount of D2O and DDW in each sample and the number of seeds in each sample as well:
And here is the setup:
- I began by preparing 8 15ml falcon tubes with the water required as shown in the table above. I used a 1mL, 2.5uL, and 100uL pipettemen to make sure the amounts were as accurate as physically possible (for me).
- I then setup 8 analyslides and poured the amount of seeds shown above into each sample chamber.
- I added the water from the first step to the chambers and then closed them.
- I then sealed the chambers with the vacuum grease and placed the samples in the fridge to synchronize their growth. I will remove them on Monday for Day 0 pictures.
It is important to note that there was a mishap with the 20% D2O in DDW sample. As I was closing the slide, about 1/3 of the water and a couple seeds leaked out (clumsy hands). I don’t anticipate much of a problem with the experiment except for the added exposure to atmosphere so at the end of the trial I will probably discount the results from that sample.
DOI: 10.15200/winn.142802.21826 provided by The Winnower, a DIY scholarly publishing platform
Like this:
Like Loading...