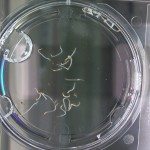
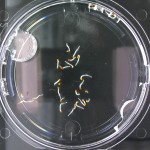
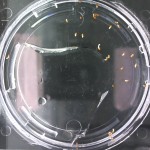
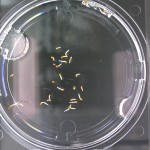
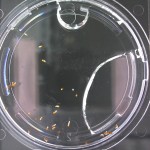
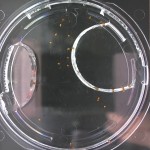
I’m leaning towards doing this trial for 2 more days because all the seeds are at/approaching max germination. Beyond day 15 I’ll keep my eye on the D2O sample to see if there is any sprouting, but it looks like it is becoming a race against evaporation.
Category Archives: RC1
Repeating Crumley: Day 11
Repeating Crumley: Day 10
Double Digit Days!
Repeating Crumley: Day 9
Nothing new to report. It looks like the 99% D2O seeds still haven’t sprouted which is what should happen (contrary to Crumley’s report). Everything else is growing as expected. And as usual there is nothing in the sample with no seeds.
Repeating Crumley: Day 8
And the days go on and on and on…
Pretty soon this experiment will be developing into something more and I’ll be looking to write the results up from this experiment in multiple formats (stay tuned). Anyways onto the notes:
- As I mentioned in my other post from today I sealed these samples with nail polish. How that works out remains to be seen, but as you can see in the 99.9% D2O sample and the 66% D2O sample this was becoming an urgency.
- Because of the movement involved with the sealing, the seeds are now in different locations then they have been in the past pictures. This presents one new challenge, but shouldn’t affect the results. It should be mentioned that I further agitated the seeds because in several samples (like the DDW sample) there were multiple bubbles and I wanted the seeds to be away from the water-air interface.
- In the DDW sample there are plenty of seeds that have shed the seed coat and this fact, along with the fact that I moved everything makes it hard to find the remaining one or two seeds that may/may not have begun germination. So until I notice little radicles I will keep the count the same. This goes for the DI Water sample as well.
- Finally make note that there is a new picture of a new sample which I also mentioned earlier. This sample is seeds in DI Water in a glass petri dish (much bigger than the analyslides) and sealed with beeswax. My folly is that I’ve discovered it is really hard to remove melted beeswax from other glass surfaces and I’ve tried boiling water and it is just not doing it. Oh woe is me!
Update: I also wanted to add one final note. I noticed yesterday that it seemed most of the seeds in the ddw sample were a little further along in their development than the seeds in the di water sample. The roots appeared longer and there are quite a number of seedlings without their seed coat (first leaves and such) in the ddw water.
Repeating Crumley: Flaws and Fixes
I’ve come across a decent flaw with my setup. It seems the airflow is a little more than expected. Well at least it is enough to notice. The air bubbles in a few samples have been getting noticeably larger, in fact in one sample there is more air than water. So in an effort to stop this I’ve done one thing and am trying a few new things:
- Upon Koch’s suggestion I attempted a seal with nail polish. I have experience with this before when working with DNA tethering. I would make a micro-channel from a slide, a coverslip, and two pieces of double stick tape. This would make a rectangular channel with two ends open, to flow DNA and other components. After all flowing was finished I would seal the chamber with nail polish. In my experience there is still some air flow, but a sample would last for days and we are talking a volume of ~12ul. Here I am dealing with a volume of 6ml which is roughly 500 times more. If I get comparable air flow, the effect could be negligible.
- As an idea, I’m trying to see if beeswax creates a decent seal. I filled the lid of a glass petri dish with melted wax and coated it. Then I poured out the wax that wasn’t solid. I filled the bottom with water and seeds and pushed the top onto the bottom. We’ll see what happens over time…
- As an aside of an idea, I’m playing with measuring the seeds via the cellattice microruled coverslips I have. I’m also seeing whether seeds can grow in nail polish. I added some polish around the rim of the cellattice slip to bond it to the inside surface of the bottom of the analyslide petri dish. Then I added a very thin coat of polish to the top of the celllattice slip and dropped some seeds on top. I let it all dry for about 30 minutes before adding water to the sample. The idea was that just slight contact with the polish should hold the seeds in place over the ruler and it worked… so far.
I’ll let you know how these little experiments work out over the next few days.
Typical Specimen for the Repeating Crumley Experiment

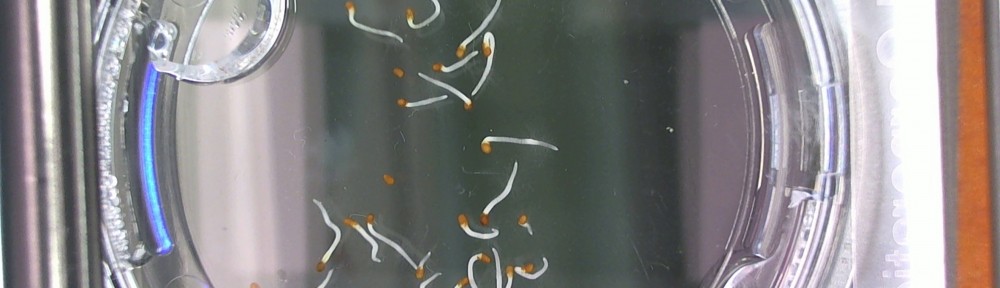
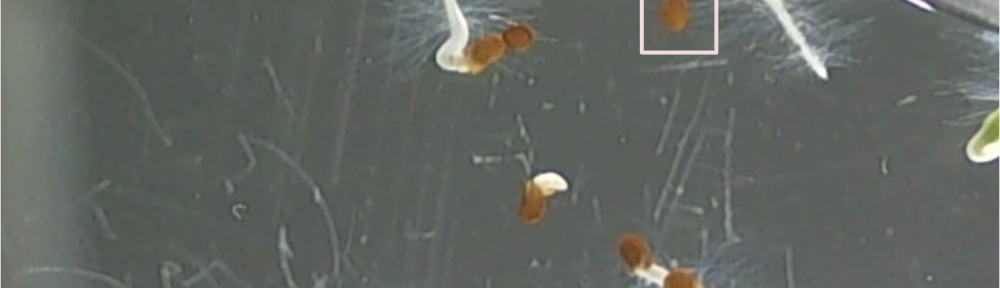
Bill Hooker suggested (and rightly so!) that I document what I consider to be typical specimens. I found, today that the zoom feature on the webcam I’m using is quite sufficient for getting close enough to demonstrate this and so I snapped a picture and tried to document. Powerpoint messed me up a little bit, but this is good enough.
The orange box is highlighting a seed that is showing no signs of germination. From my studies I’ve found that the the seed coat becomes slightly transparent just before radicle (the pre root) penetration and you can see the precursor to puncture. The seed coat usually gets lighter too and in this case is really dark in color.
The pink box (hehe) is what I would count as germination when I’m counting the seeds. I see the tip of the radicle penetrating the seed coat and so germination is officially underway.
In the very near dead center of the image is a seed that has what I referred to in an earlier post as an extended radicle. Basically I meant that the radicle was pushed through and the root is now forming. The root will continue to grow until the seed coat has come off, which over to the right it has (there is a green leaf that is cropped on the edge of the image).
The end.
DOI: 10.15200/winn.142721.16402 provided by The Winnower, a DIY scholarly publishing platform
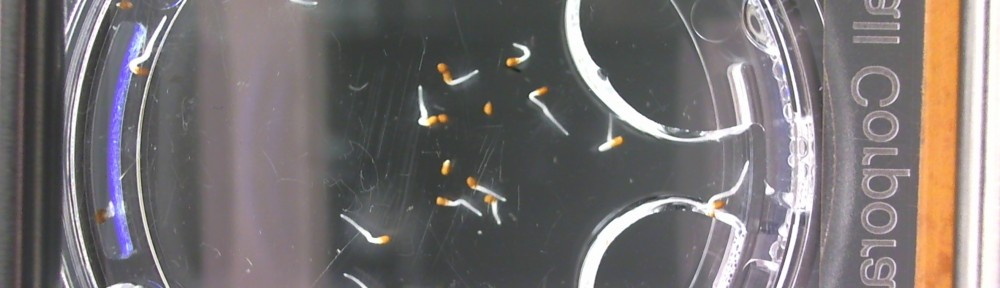
Repeating Crumley: Day 7
Here are the Day 7 results. Looks good so far. There are some troubles though. It looks like I have extensive leakage in the 66% D2O and 99.9% D2O samples, because each day the air bubbles get bigger. How can I fix this without restarting the experiment?
Also in the 33% D2O sample there is that white thing near the center and two unsprouted seeds. I have a feeling the white thing may either be from one of those two seeds. It could also be from a broken seed from the pack that was stuck to the tweezers and which I didn’t notice got added to the lot when I poured the seeds into here. What to do? I won’t know for sure until like Day 15 or so when most of the seeds in this sample would have sprouted. Ideas?
Finally you can definitely see the root hairs in the DDW sample and the DI water samples this time. Not so much in the 33% sample. In all honestly I can’t even tell by the naked eye. Hopefully this will be obvious by day 10. And as usual, nothing to report in the no seed sample.
Repeating Crumley: Day 6
Here are today’s results.
And the notes: All seeds showing advanced germination (meaning longer than just a white tip) by my eye exhibit some form of root fro. As per usual it is most prevalent in the deuterium depleted water but this time the di water samples aren’t far behind. While it isn’t noticeable yet in 66% D2O samples, there is some root fro in the 33% samples, but I think it is too soon to tell if the fro compares with that in the ddw/di water samples because the germination isn’t as far along in that sample compared to the others. Right now I’d say the fro’s are just puffs.
It was suggested by Bill Hooker in the comments for Day 5’s results that I point out what I am determining is sufficient germination for the seed to be counted. If you look at the 66% D2O sample there are three seeds towards the bottom with white tips (not close to each other) and I would say that is sufficient germination for sure. That is probably the make or break point. Any less than that and it’s hard to tell if it’s germinating or if the seed coat is just becoming a lighter color, which apparently it does once it starts absorbing water.
Repeating Crumley: Day 5
Two very important things to note:
- There is still nothing growing in the sample with no seeds.
- I’m not sure when to start counting the seeds in 66% D2O, it seems that there are definitely some seeds that are showing initial signs of germination, but nothing obvious (like an extended radicle). I’ll compare this picture to the day 3 images when I considered those seeds to have begun germintion.
- Also note that yesterday I didn’t look at the seeds until around 5pm, and today I’m looking at them at 10am. Obviously much less time has passed between now and yesterday than yesterday and the day before.