Time for my weekly Time Trial experiment. This time I pit the growth of yeast in 20% D2O vs yeast grown in 40% D2O.
Category Archives: D2O Adaptation 3
Yeast in 20% D2O
Yeast in 20% D2O Time Trial 2 Live Results
20% D2O YPD Agar Plate
I made 2 20% D2O Agar Plates:
- 32ml of DI YPD
- 8ml of D2O YPD
- 0.8g agar
- stirred and heated until agar dissolved
- 15-20ml of agar solution into petri dishes
I allowed the plates to cool and sealed one and put it in the fridge for later use. The other went streaking and is in the incubator at 30C.
D2O Adaptation Live Results
Here are my daily results. Each week I will be increasing the % of D2O in solution, starting at 20% D2O.
Yeast in 20% D2O Time Trial
Setup. Today I”m doing the time trial and I setup 3 samples, following my protocol:
- Yeast in DI YPD
- Yeast in 20% D2O YPD
- Yeast in 99% D2O YPD
Live Results:
DI YPD Yeast Observation
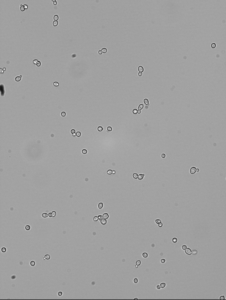
I needed an image of wt yeast in DI YPD to compare with the morphology of later strains. As I ramp up the D2O concentration, I expect to see some differences and this is good information to have in general.
I don’t think there is much to infer from the 20% D2O comparison image, but if it’s worth anything there are a few cells in that image that are elongated, and I never saw that morphology in DI water.
D2O Adaptation 3 Starter Culture
Today is the start of the starter culture. It should be ready for inoculation by Friday morning.
D2O Adaptation Try 3 – Project Plan
Now that the lab is clean, I’m going to collect my thoughts and write a plan to follow. While the first two experiments were unsuccessful, there were some interesting results that can’t be disregarded. But in the pursuit of science more results are required! So let’s get this party started…
D2O adaptation: Based on the paper I read yesterday, it seems that a slow adaptation is the right way to go. Yeast grown in pure D2O seemed to endure too much stress to adapt and the adaptation might be way slower compared to a progressive adaptation plan. So each week I’ll increase the amount of D2O by 20% starting with 20% D2O YPD:
- 20% D2O
- 40% D2O
- 60% D2O
- 80% D2O
- 100% D2O
Plan:
- Every Monday I plan on running a time trial experiment comparing the growth of yeast in DI YPD to the increased D2O YPD concentration and to the previous D2O concentration.
- Every Friday I’ll run a time trial of the yeast growth in that week’s D2O concentration.
- Every day, yeast will be provided new medium for growth and daily nanodrop readings will be recorded to analyze the 24 hour growth. Also a microscope analysis will also be done to ensure there is no contamination.
- Weekly glycerol stocks will be created in case of contamination.
- Agar plates of each D2O concentration will be created to analyze colony morphology. Inoculation onto solid media will take place each week, and right now I’m not sure when the best day to do this is, but I’m thinking Wednesdays might be best.
Daily Yeast Growth Protocol
These are my protocols for the D2O adaptation experiments. I’m making this post so I don’t need to write a post daily unless something out of the ordinary happens.
Making YPD:
- Use autoclaved beakers and bottles for water handling and storage.
- Measure water volume: D2O usually is supplied as a little over 90ml per bottle, DDW is about 100ml per bottle, and DI is readily available in whatever quantity needed.
- Measure YPD powder and add 5g per 100ml of water.
- Add a clean stir bar and stir (using hotplate/stirrer) until YPD is fully dissolved. For D2O and DDW stir at a low speed to minimize air mixture with solution.
- Using a syringe and a filter, filter the YPD into the bottles. This step is necessary for D2O and DDW YPD to ensure minimization with H2O/D2O contamination. For DI YPD autoclaving is sufficient.
- Label bottles and date.
Daily Prep:
- For starter cultures, add 10ml of YPD to an autoclaved test tube. For daily measurements, add 9ml of YPD.
- Inoculate yeast in liquid media. For daily measurements, inoculate 1ml of culture from the previous generation’s water type (ie add 1ml of 99% D2O yeast to 99% D2O YPD).
- Add to incubator/shaker and incubate at 30C and 185RPM.
Nanodrop Measurement:
- Aliquot a minimum of 400ul of sample into semi-micro cuvettes. Normally I measure at 0 hours and at 24 hours (or every 24h thereafter for prolonged experiments) for daily measurements and hourly starting with the 0h measurement for time trial experiments.
- Blank the nanodrop with pure YPD and make sure to press the cuvette checkbox (I always forget this!).
- Measure each sample.