E. Coli Growth over 4 hours. Anthony Salvagno, Alexandria Haddad. Figshare.
Retrieved 20:17, April 20, 2012
hdl.handle.net/10779/b627469dabcd4034053cc53040d4dcbd
I went through the data that I posted on Tuesday and realized it was even less useful to people than I expected. I almost didn’t even know what I was looking at! Anyways, I did a couple of plots in excel with the data (which can be found on FigShare along with both the original data and the revised and cleaned data) and tried to extrapolate some other information. But first let’s discuss the experimental setup.
So on Monday, Alex created a starter culture from the E. coli we grew on plates last week. Then on Tuesday (I realize how not very real-time this post is for me, but the data came out in real-time which is also important) we made 3 dilutions of the starter culture to track the growth of the E. coli over time. We did:
- 1ml in 9ml of LB broth (1:10)
- 2m in 8ml of LB broth (1:5)
- 5ml in 5ml of LB broth (1:2)
Every hour we took an absorbance reading from the nanodrop and read the 600nm value (A600 according to the machine). We also reblanked every hour according to the instructions from the nanodrop. The initial readings were:
- starter culture – 1.076
- 1:10 – 0.097
- 1:5 – 0.23
- 1:2 – 0.665
It is interesting to note (and I literally just noticed this), that the initial readings are almost exactly what the dilutions are, ie 0.097 is ~1/10 of 1.076. Good for us!
In the FigShare data, you will find the original data (which I linked to in my post on Tues, but in Google Docs instead of Excel) and a revised data set. The data is messy but the graphs are interesting.
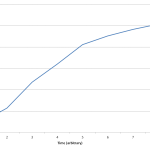
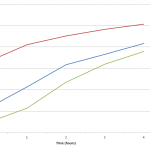
Also I tried to link the 3 data sets together into one coherent graph, but the time series doesn’t seem to match up right, or maybe it does and I just think it doesn’t. The 1:5 dilution seems to provide a bridge between the data in the 1:10 dilution and the 1:2 dilution. After about 2 hours the 1:10 sample overlaps with the 1:5 and likewise the 1:5 begins to overlap with the 1:2. Also at hour 3, the 1:10 sample overlaps with the 1:2 sample.
Because of this I tried to graph the data as one continuous set. It seems Like it may be alright, but I feel that the in the 1:2 sample there isn’t much growth in the 4 hours, which is reflected in the 3 data set plot, but it doesn’t look like it peaks in the continuous graph. Hmmm… Anyways check out the FigShare data.
PS I’m including the two plots I’m referring to below.